TOPIC 5: VOLUMETRIC ANALYSIS | CHEMISTRY FORM 3
VOLUMETRIC ANALYSIS
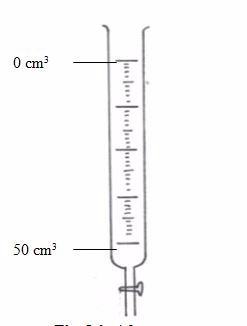
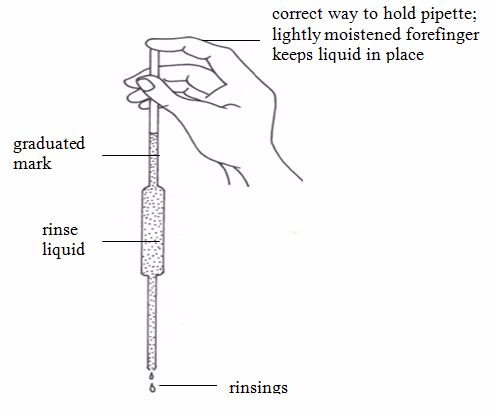
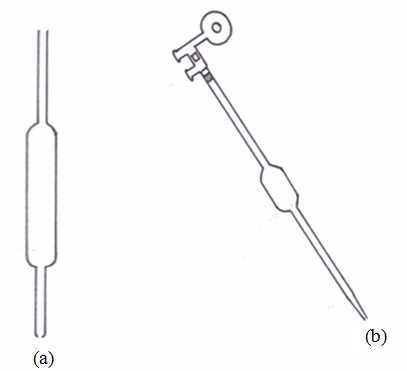
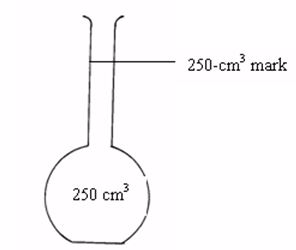
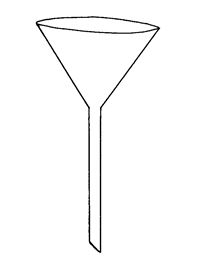
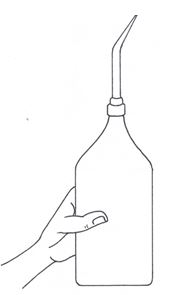
Standard Volumetric Apparatus
Procedure
<> Use in preparation of standard solutions:
Standard solutions are prepared by applying the knowledge of volumetric analysis. Volumetric analysis is used in school, college and university chemistry laboratories to determine concentrations of unknown substances.
The titrant (the known solution) is added to a known quantity of analyte (unknown solution) and a reaction takes place. Knowing the volume of the titrant allows one to determine the concentration of the unknown substance.
<> Use in environmental and water safety
Titration is important in environmental chemistry, where scientists can use it to analyze acid rain or contaminants in surface water samples. Environmental studies usually involve an analysis of precipitation and its response to pollution.
To quantify the degree of contamination in natural rainwater or snow, titration is used. The process is quick and results are reliable. Since most titration processes do not require expensive or specialized equipment, the test can be performed often and in different areas with relatively little effort.The safety of water is based on its chemical ingredients.
By analyzing wastewater, the extent of contamination and the requirements for filtering and cleaning can be determined. Titration is a key mechanism in this analysis. Often, more specialized titration equipment is used in this application, which measures ammonia levels in combination with other reactants to quantify other chemicals present.
<> Use in food and beverage industry
In the food and beverage industry, manufacturers must ensure their products meet certain quality criteria or contain standard concentrations of specific additives, so titration is often used to analyze the products before sale. Wine is often affected by its degree of acidity.
It is possible to improve wine production by measuring acidity using titration. Simple, inexpensive titration kits are available to winemakers for this purpose. The results of a titration test on wine can suggest if additional ingredients are necessary to maintain its quality.In general, all brewing industries and distilleries apply the knowledge of volumetric analysis (titration) to determine the acidity and alcohol contents of their beers and other alcoholic beverages.
The process also finds ample use in food industry. The compounds which make up food products help determine their nutritional implications. Titration is one technique that assists in these studies. The acidity of orange juice, for example, is easily determined using a standard titration process.
In this process, an electrode is added to a solution made up of orange juice and deionized water. The titrant catalyst then measures the acidity of the juice. Manufacturers can use the technique to vary this quality to satisfy customers or those with special nutritional needs.
<> Use in agriculture
Volumetric analysis technique is used to determine the soil pH. This is important because, if the pH of a certain soil is found to be extremely low or high, corrective measures are taken by adding the correct quantity of agricultural limes or other chemicals to make the soil suitable for plant growth.
The method is also used by agronomists and farmers to analyse the kind and amount of plant nutrient elements present in a particular sample of soil, the knowledge of which helps determine soil fertility.
Industrial and Laboratory Skills of Volumetric Analysis
Compare industrial and laboratory skills of volumetric analysis
The knowledge of volumetric analysis (titration) is used in hospitals and medical laboratories to carry out such duties as preparation of solutions and suspensions, blood analysis, and diagnosis of certain diseases and health problems.
For example, when dissolving a solid drug to make a solution for injection, utmost precision is required to measure the correct volume of liquid to be used to dissolve a correct amount of solid drug to prepare the solution of a given concentration to inject to a patient.
Also titration is very important in the pharmaceutical industry, where precise measurements of quantities and concentrations are essential throughout the manufacturing process.
Titration is thus an important part of the pharmaceutical industry to ensure quality control. Many variations of the titration technique are used, and specialized equipment for pharmaceutical titration is often developed to make the process more efficient.













































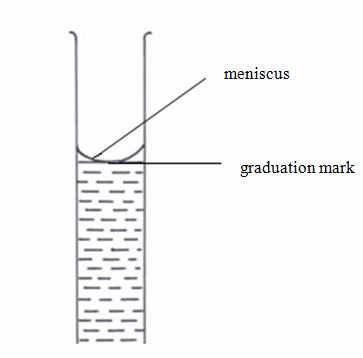
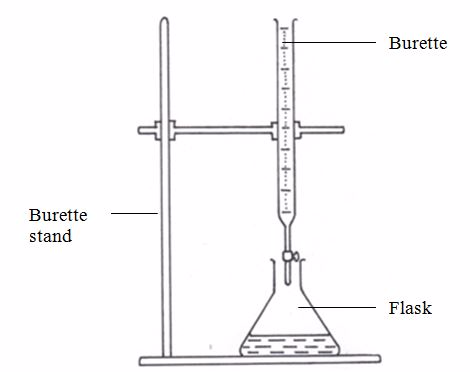
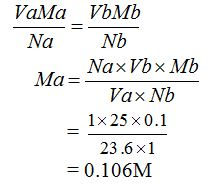
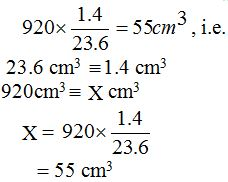
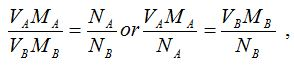
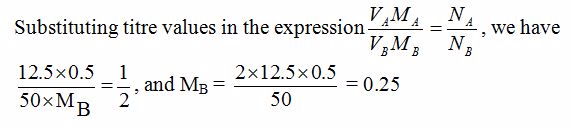
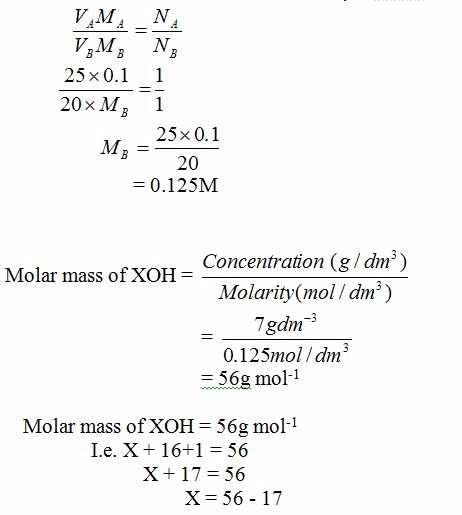
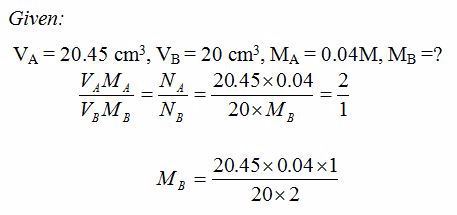

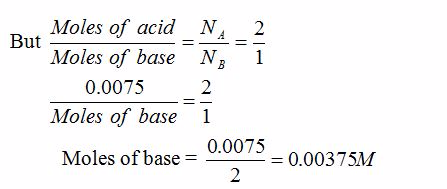
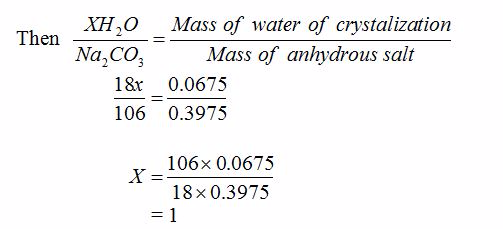











[url=http://cheapdrugs.store/#]ed dysfunction[/url]
All questions must be solved